In April 2020, the U.S Food and Drug Administration (FDA) issued a recall for the heartburn medication Zantac, after carcinogenic impurities were found in samples of the prescription drug. The recall, along with a surge in reports of serious complications caused by the drug, has led to well over 100 lawsuits against drug manufacturers – Sanofi S.A. in particular. These lawsuits could soon number in the thousands.
What Is Zantac and What Is it Used for?
Zantac is a prescription drug that treats and prevents heartburn – a condition that affects 15 million Americans daily and 60 million Americans at least once a month. Zantac’s generic name is ranitidine and has also been available over the counter.
Ranitidine is a part of a group of drugs called histamine-2 blockers that decrease the amount of acid your stomach makes. It is most commonly used to relieve heartburn – a burning pain or sensation in the chest that occurs when acid is backed up into the esophagus. If heartburn persists, it could be a symptom of a more serious and chronic condition of acid reflux: Gastroesophageal reflux disease (GERD) or an ulcer in the stomach or intestines. Ranitidine treats both conditions.
Why Was Zantac Recalled?
As early as 2018, European and American regulators began finding low levels of N-nitrosodimethylamine (NDMA) in some samples of ranitidine. NDMA is classified as a probable carcinogen or a chemical that can cause cancer. Low levels of NDMA can be found in food and water, and have not raised concern. However, there is a possibility that “sustained higher levels of exposure may increase risk of cancer in humans,” according to the FDA news release.
In June 2019, the online pharmacy company Valisure initially notified the FDA that they had found low levels of NDMA in some of the batches of ranitidine. After a safety review, the FDA released a statement alerting the public of the findings of NDMA in September 2019 – still limited at this point.
Shortly after, the pharmaceutical company Sandoz issued a voluntary recall for ranitidine and major pharmacy chains including Walgreens, CVS, and Rite Aid stopped selling Zantac and its generic versions.
On April 1, 2020, the FDA recalled all products of ranitidine from the market, both prescription (brand name Zantac) as well as over-the-counter and generic versions. New FDA findings were more definite, and they confirmed that NDMA levels in ranitidine increased notably when stored in high temperatures, but also, under normal storage conditions. This meant that NDMA levels increased in temperatures the drug was stored in during distribution or consumer use. In addition, the findings determined that the level of NDMA level depends on when the ranitidine was manufactured – the older the ranitidine, the higher the NDMA levels.
Zantac Forms Recalled by FDA
Date of Recall Product RecalledCompany2/27/20Ranitidine Tablets, USP 150mgAmerican Health Packaging1/8/20Ranitidine Tablets 150mg and 300mgDenton Pharma, Inc. dba Northwind Pharmaceuticals1/7/20Ranitidine Tablets 150mg and 300mgAppco Pharma LLC12/17/19Ranitidine Tablets 150mg and 300mgGlenmark Pharmaceuticals, Inc.11/22/19Ranitidine Tablets, 150 mg and 300 mg, and Ranitidine Syrup (Ranitidine Oral Solution, USP), 15 mg/mL Amneal Pharmaceuticals, LLC11/19/19Ranitidine Oral Solution, USP 150 mg/10 mL Precision Dose Inc.11/15/19Ranitidine HCl 150mg and 300mg Capsules GSMS, Inc.11/8/19Ranitidine Liquid Unit Dose Cups American Health Packaging11/6/19RanitidineAurobindo Pharma USA, Inc.10/25/19Ranitidine Hydrochloride Capsules 150 mg and 300 mg Novitium Pharma10/25/19Ranitidine Syrup (Ranitidine Oral Solution, USP), 15mg/mL Lannett Company, Inc.10/23/19Ranitidine Tablets & Capsules Dr. Reddy’s Laboratories Ltd.10/23/19Zantac 150, Zantac 150 Cool Mint, Zantac 75 (OTC Products)Sanofi10/23/19Ranitidine (all pack sizes)Perrigo Company plc9/25/19Ranitidine Tablets 75mg and 150mgApotex Corp.9/23/19Ranitidine Hydrochloride Capsules Sandoz Inc.
History of Zantac Manufacturers
The pharmaceutical company Glaxo Holdings Ltd, now a part of GlaxoSmithKline PLC, brought Zantac to the market in 1983, after receiving approval from the FDA. In just five years, Zantac gained popularity and became a world best-seller – exceeding $1 billion in annual sales for the first time. From 1987 to 1988, Glaxo’s annual sales rose 37 percent, reaching $1.3 billion and naming Glaxo the world’s second-largest pharmaceutical company.
In 2004, over-the-counter versions of Zantac became available. In 1997, Glaxo’s patent for ranitidine expired in the U.S., and competitors began developing generic versions of Zantac.
In 2004, the pharmaceutical company, Pfizer received approval from the FDA to sell over-the-counter versions of Zantac in the U.S. From there, Pfizer licensed the drug to other companies, including Johnson & Johnson and Boehringer Ingelheim Pharmaceuticals. Sanofi S.A., the most current seller of Zantac, purchased it in 2017. In 2018, Sanofi reported it made roughly 124 million in U.S. currency.
Zantac Prescription and Serious Side Effects Statistics
According to the FDA Adverse Event Reporting System (FAERS), they received a total of 73,240 cases with a negative effect of Zantac since 1983.* This includes:
- 55,891 serious cases
- 4,926 deaths
- 66 percent of the total cases were linked to cancer
*This number combines cases where the patient had multiple adverse reactions. For example, if a patient reported three different reactions, we counted them just once
In 2018, 18,739,686 people in the United States were prescribed Zantac. The same year, 1,094 cases involving Zantac were reported to the FDA. There were 316 cases that reported that the drug was ineffective. At this time, only two cancer cases were identified.
Sales and Marketing Costs
Over the years, pharmaceutical companies have earned the reputation of spending more money on sales and marketing over research development –– with the majority of expenses targeted towards physicians. In 2012, the pharmaceutical industry spent $24 billion on marketing to physicians and $3 billion on advertising to consumers, primarily through television commercials. Pharmaceutical companies play a significant role in which drugs physicians choose to prescribe to their patients. A study showed that when doctors received payment from pharmaceutical companies, they were twice as likely to prescribe their drugs than those who did not. Another study showed that payment increased prescription of the drug by 73 percent.
In 2013, Johnson & Johnson spent $17.5 billion on sales and marketing – more than double the amount they spent on research development, according to the healthcare research firm GlobalData. Pfizer spent $11.4 billion on sales and marketing, compared to $6.6 billion on research development.
From 2012 to 2016, prescription drug advertising expenses increased by 62 percent – specifically for direct-to-consumer advertising, or TV ads.
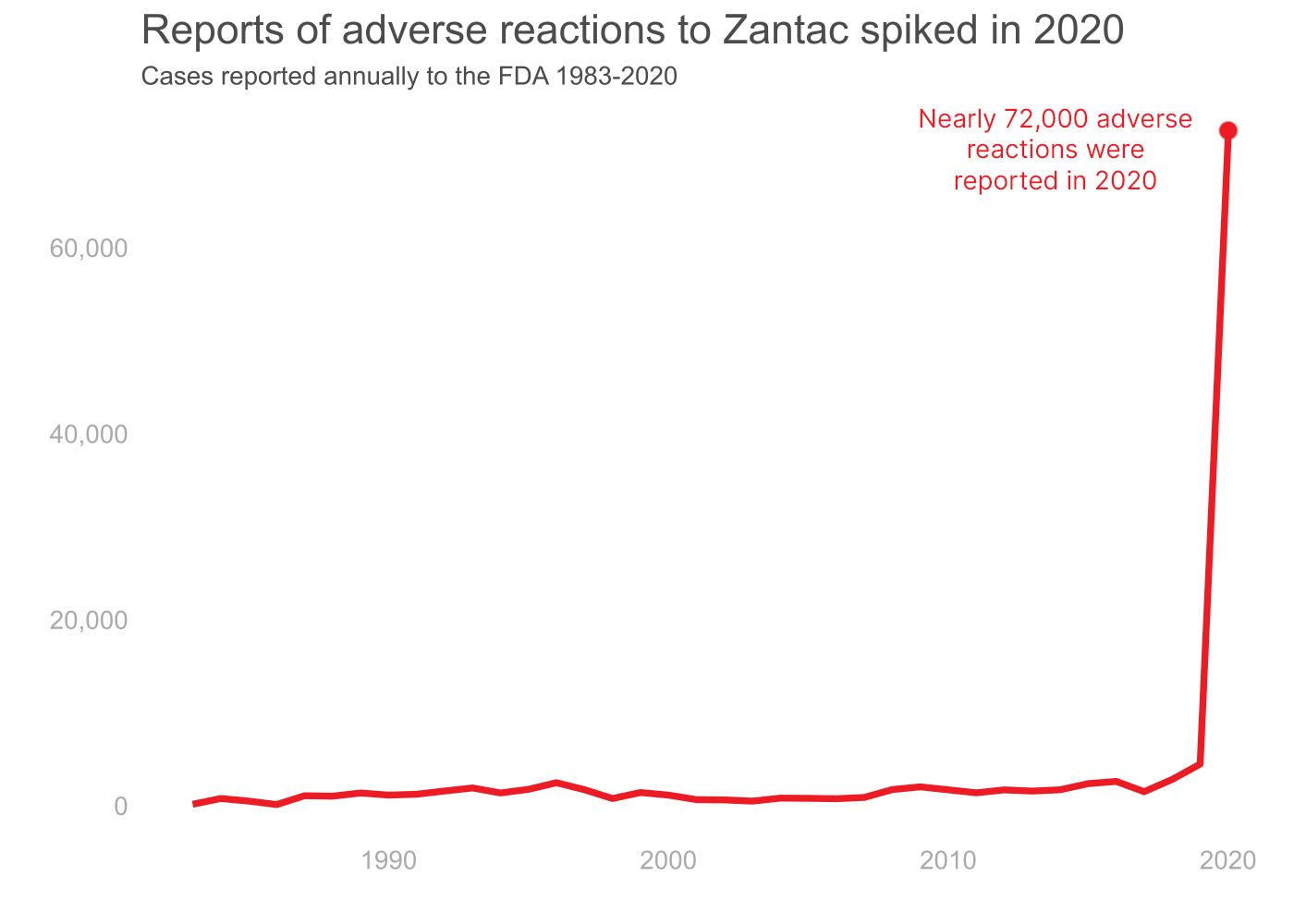
Zantac Adverse Reaction Cases by Year
The FDA received the majority of cases in 2020: 71,861, the first year after the recall. Out of those, 69,150 cases were serious.
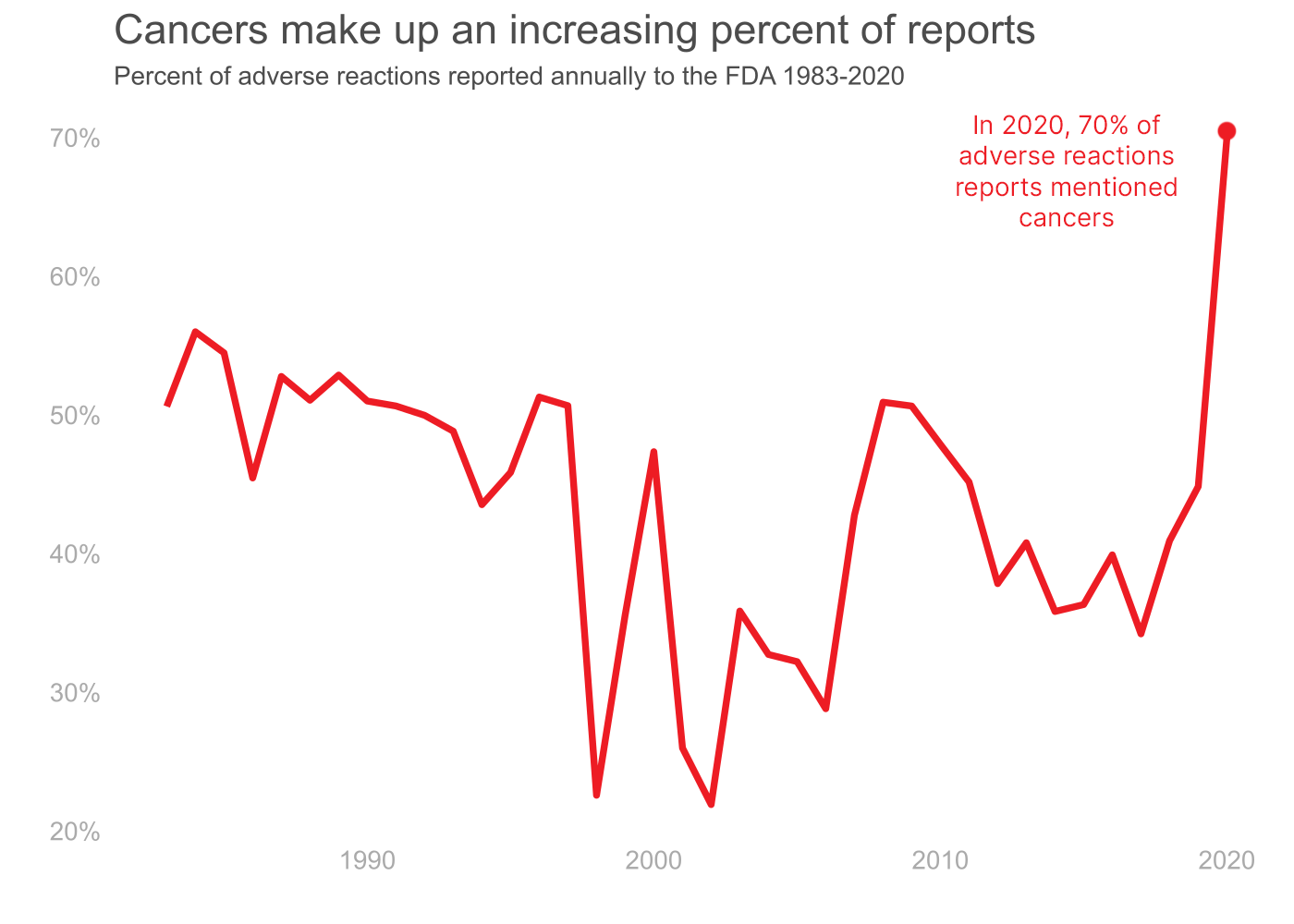
Zantac Cases and Link to Cancer
We looked at the total number of reactions reported to the FDA from 1983-2020 and found that cancer cases made up an increasing percentage of reports. Out of the 73,240 cases, 66 percent were linked to cancer. In 2020, 70 percent of reactions reported were cancers.
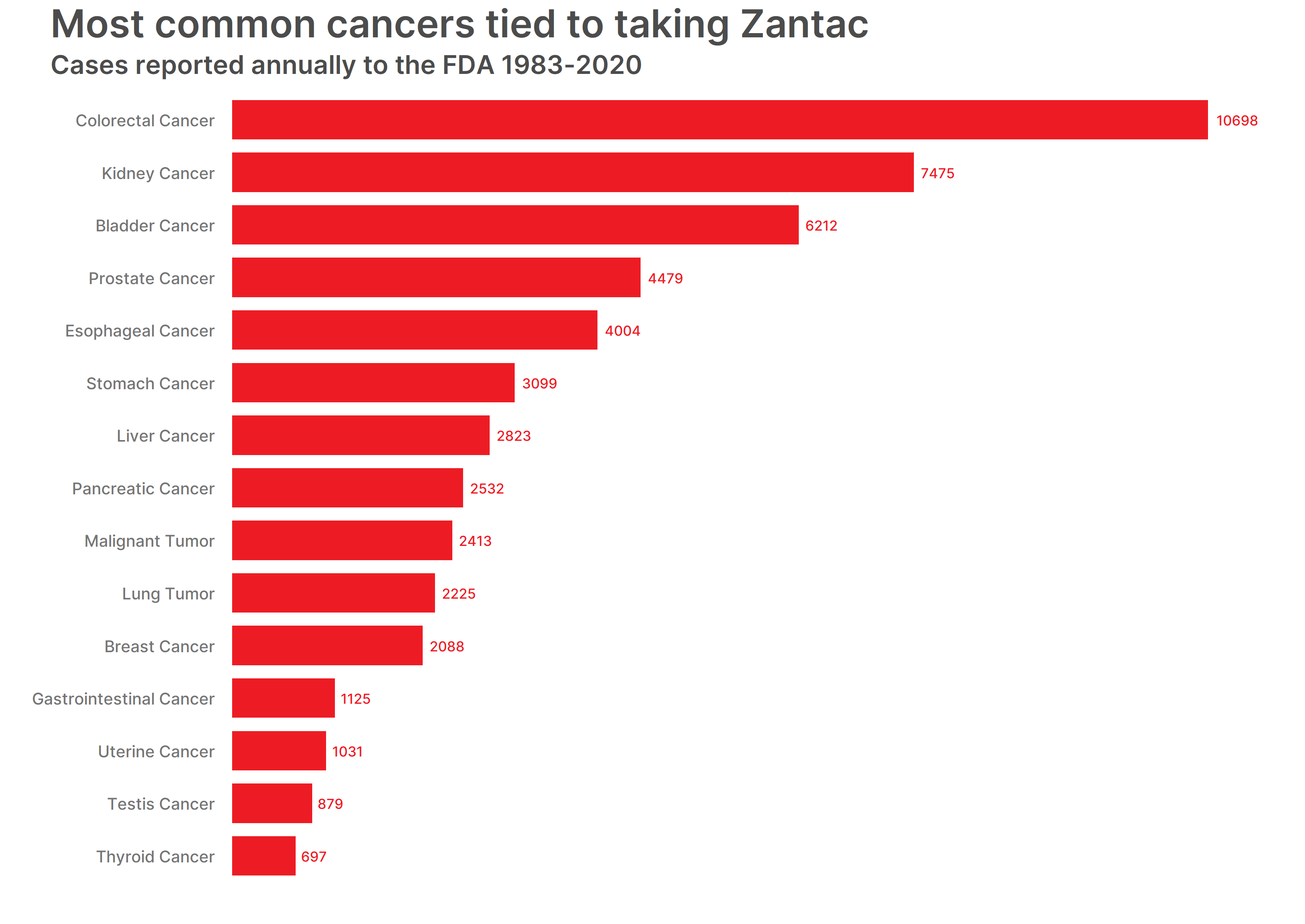
The most common cancer listed as a reaction was colorectal cancer – or colon cancer. Patients reported 10,698 colon cancer cases after taking Zantac. Kidney cancer and pancreatic cancer were also listed in the most common reactions tied to Zantac and had increased rates of new cases overall. The rate of new cases for kidney and renal pelvis cancer showed a steady increase from 1992 to 2008 and remained high from 2008 to 2018. The rate of new cases for pancreatic cancer steadily increased from 2004 to 2018.
The top 15 cancers listed as reactions in total Zantac cases are:
- Colorectal Cancer – 10,698 cases
- Kidney Cancer – 7,475 cases
- Bladder Cancer – 6,212 cases
- Prostate Cancer – 4,479 cases
- Esophageal Cancer – 4,004 cases
- Stomach Cancer – 3,099 cases
- Liver Cancer – 2,823 cases
- Pancreatic Cancer – 2,532 cases
- Malignant Tumor – 2413 cases
- Lung Tumor – 2225 cases
- Breast Cancer – 2088 cases
- Gastrointestinal Cancer – 1125 cases
- Uterine Cancer – 1031 cases
- Testis Cancer – 879 cases
- Thyroid Cancer – 697 cases
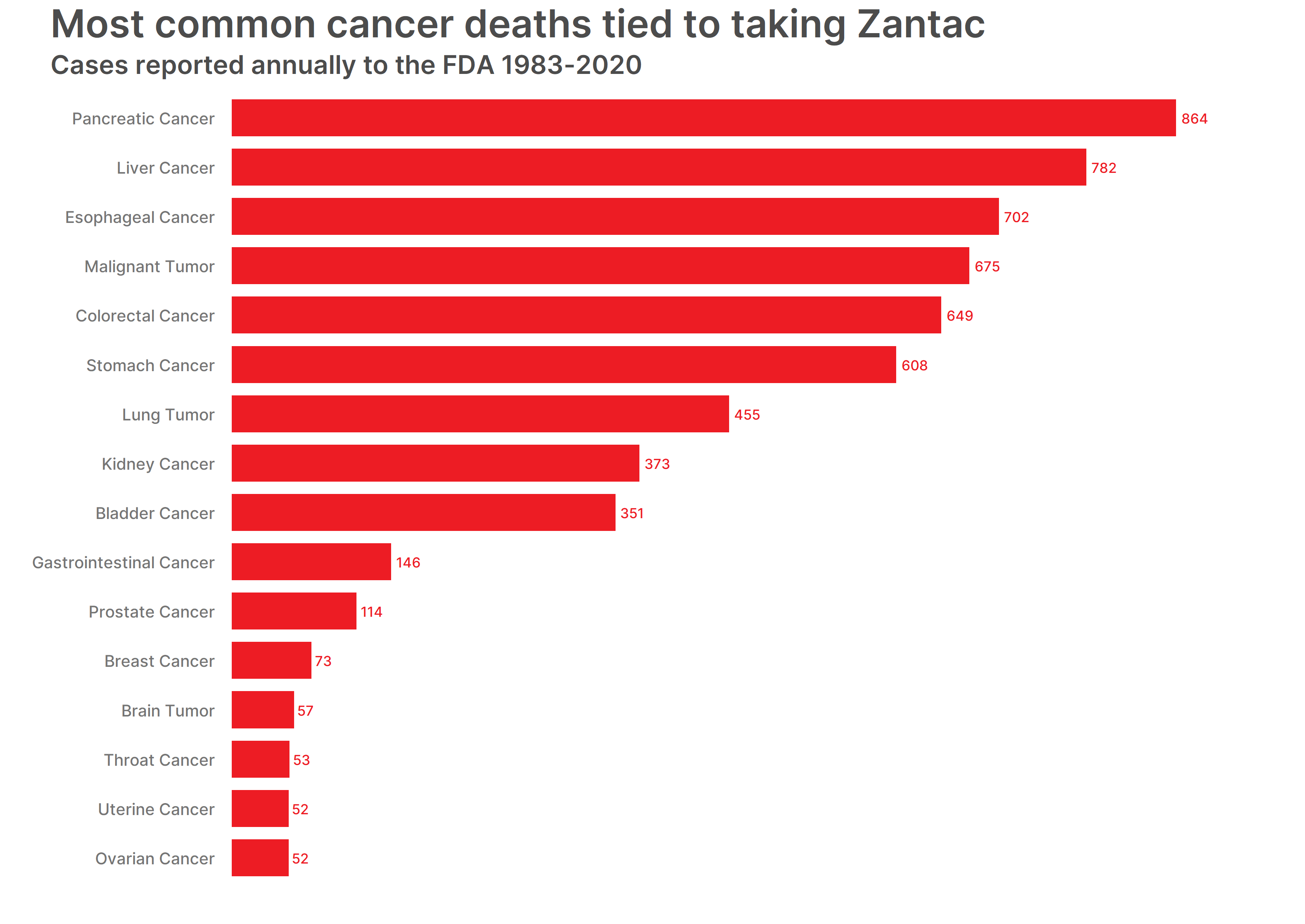
We also compared Zantac cases that led to death and the reactions listed. In 87 percent of the death cases, cancer was the leading cause. Pancreatic cancer resulted in the most Zantac death cases, followed by liver cancer and esophageal cancer.
The top 15 cancers listed in Zantac death cases are:
- Pancreatic Cancer – 864 cases
- Liver Cancer – 782 cases
- Esophageal Cancer – 702 cases
- Malignant Tumor – 675 cases
- Colorectal Cancer – 649 cases
- Stomach Cancer – 608 cases
- Lung Tumor – 455 cases
- Kidney Cancer – 373 cases
- Bladder Cancer – 351 cases
- Gastrointestinal Cancer – 146 cases
- Prostate Cancer – 114 cases
- Breast Cancer – 73 cases
- Brain Tumor – 57 cases
- Throat Cancer – 53 cases
- (tie) Uterine Cancer, Ovarian Cancer – 52 cases
Deadliest Cancers Linked to Zantac Use
While we knew that cancer was the leading cause of death in Zantac death cases, we wanted to find out which cancer type was the deadliest. It makes sense that pancreatic cancer – the cancer type tied to most Zantac deaths – was the deadliest cancer. Pancreatic cancer resulted in 34 percent of patients’ deaths. Other deadly cancers included a malignant tumor, liver cancer, and brain tumor. They caused the deaths of nearly 30 percent of patients.
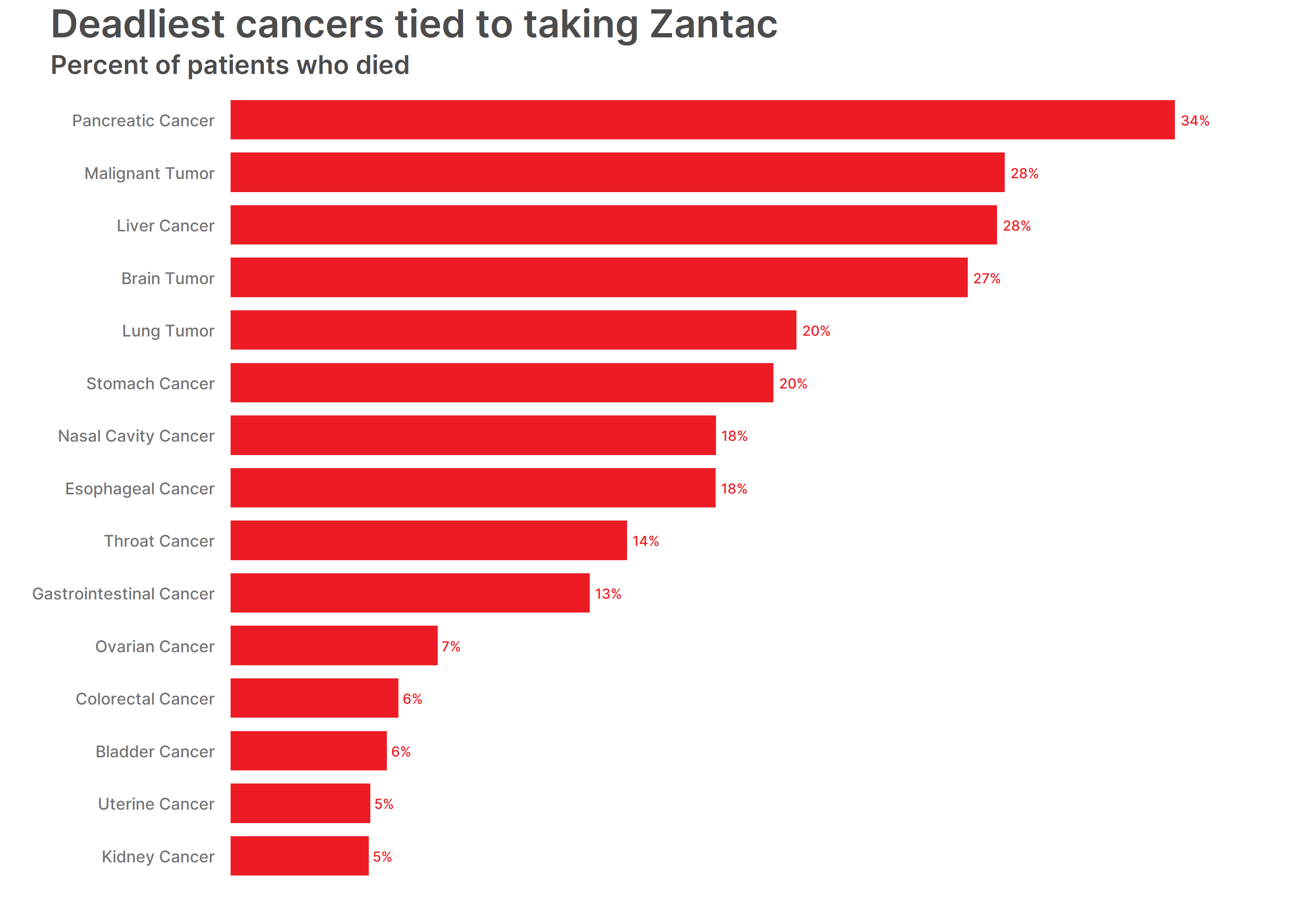
Pancreatic Cancer – 34%
Malignant Tumor – 28%
Liver Cancer – 28%
Brain Tumor – 27%
Lung Tumor – 20%
Stomach Cancer – 20%
Nasal Cavity Cancer – 18%
Esophageal Cancer – 18%
Throat Cancer – 14%
Gastrointestinal Cancer – 13%
Ovarian Cancer – 7%
Colorectal Cancer – 6%
Bladder Cancer – 6%
Uterine Cancer – 5%
Kidney Cancer – 5%
Zantac Injury Demographics
The majority of cases did not specify gender or age. While anyone can experience symptoms of heartburn or acid indigestion, people who are overweight, pregnant, or elderly are more prone.
Out of the total cases of 73,240, the breakdown of cases by gender:
- Female: 12,982 cases
- Male: 8,437 cases
- Not specified: 51,821 cases
Out of the total cases of 73,240, breakdown of cases by age:
- 0-1 month: 153 cases
- 2 months-2 years: 532 cases
- 3-11 years: 295 cases
- 12-17 years: 203 cases
- 18-64 years: 19,027 cases
- 65-85 years: 13,168 cases
- More than 85 years: 860 cases
- Not specified: 39,002 cases
Delays in Reporting
We wondered how long it took patients to report their reactions after taking Zantac and found that the more serious the reaction, the longer it took to report. In general, there was a delay in reporting Zantac-related cancers – as cancer can take years to develop. We found that patients took around four years to report cancer, around two years to report anxiety and much less time to report reactions like itching, which were more easily discernible. Patients were also quick to report if the medication wasn’t working at all.
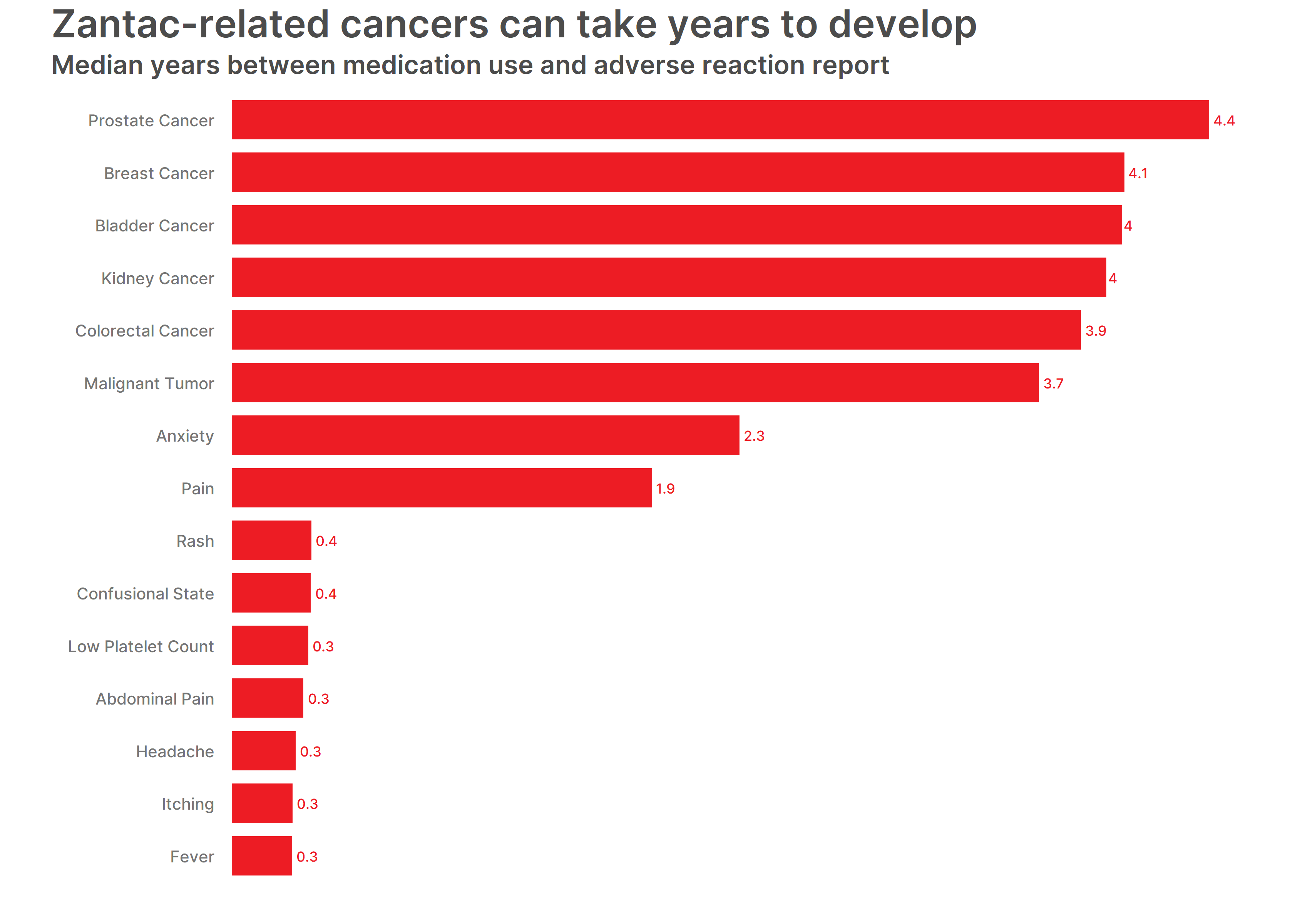
- Prostate Cancer – 4.4 years
- Breast Cancer – 4.1 years
- Bladder Cancer – 4 years
- Kidney Cancer – 4 years
- Colorectal Cancer – 3.9 years
- Malignant Tumor – 3.7 years
- Anxiety – 2.3 years
- Pain – 1.9 years
- Rash – 0.4 years
- Confusional State – 0.4 years
- Low Platelet Count – 0.3 years
- Abdominal Pain – 0.3 years
- Headache – 0.3 years
- Itching – 0.3 years
- Fever – 0.3 years
Why This Information Matters
Approximately 15 million people were prescribed Zantac annually before the drug’s link to cancer was discovered. We hope that this data provides you with the information you need. If you or a loved one has suffered a serious health complication as a result of Zanac, you are not alone. The attorneys at Shapiro Legal Group can help you seek justice and financial compensation for your losses.
Methodology and Fair Use
The data used in our analysis comes from the FDA Adverse Event Reporting System (FAERS) from 1983 to 2020. It includes a search of the following products:
- Zantac
- Zantac 75
- Zantac 150 (Ranitidine Hydrochloride)
- Zantac 150 Maximum Strength
- Maximum Strength Zantac
We combined cases where the patient had multiple adverse reactions; therefore, if a patient had reported three different reactions, we counted them once. However, certain factors – including how long the drug was used by a patient – are unknown. FAERS data consists of voluntary reporting. Healthcare professionals, patients, manufacturers, and other consumers can submit reports directly to FAERS. If a consumer or healthcare professional sends a report to the manufacturer, the manufacturer is required to send the report to the FDA.
If you would like to report or republish any of the data or images included in this analysis, please provide credit to Shapiro Legal Group by linking to this page.